Does Folding@home work?
It’s worth noting that the concept of having this level of processing power available for protein folding was unprecedented until Folding@home started in 2000, and computational protein simulation was then (and still is) a new science. How could Stanford prove that it could yield reliable and useful data?As any medical expert will tell you, the key to proving your research is often found in double-blind tests. In a medical trial, for example, a double-blind test means that neither the patient nor the person administering the drug know whether the patient is being given a placebo or the genuine medicine.
This is what Folding@home needed to prove to the scientific community, and Martin Gruebele, professor of chemistry, physics, biophysics and computational biology at the University of Illinois, was happy to be the other ‘blind’ partner. Gruebele had a research lab for experimental protein folding tests, so he and Pande could assess how the computational and experimental results compared.
"Vijay and I happened to attend the same conference at an American Chemical Society meeting," Gruebele explains, "and he was just getting his Folding@home computer system running, so we said let’s do a double-blind study. We’re not going to tell you the experimental numbers, and you’re not going to tell us what results you’re getting from the simulation. Let’s run both projects for a while in parallel, and see how they compare."
Gruebele points out that the term ‘double-blind’ should be in quotation marks, as there were no rigorous controls on the procedure, but neither scientist knew the other’s results until they were compared. The protein used for the test was based on an artificial protein designed by Barbara Imperiali’s research group at the Massachusetts Institute of Technology. Called BBA (one of a series of proteins called BBA, BBB, BBC and so on), the protein formed a part of what’s known as a zinc finger.
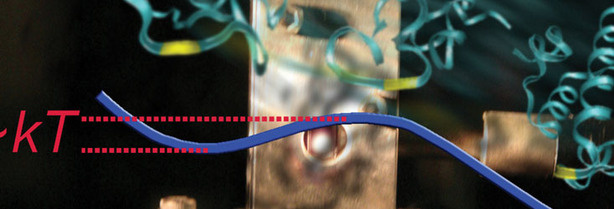
This composite image shows an experimental cell holding proteins during a fast folding measurement (the metallic block with the hole in the centre), and a protein structure folding nearly 'downhill' from left to right. The blue energy curve shows that the folded state is lower in energy than the unfolded state. Grubele is studying the impact of pressure on this protein, and is in talks with Pande about simulating this pressure using Folding@home.
"We looked at these sequences and decided that one of them could be a very good small protein for a direct comparison between computer simulation and folding," says Gruebele. "Because it’s very small, it might fold quickly." Gruebele’s lab also decided to add tryptophan to the protein to make it fluoresce under the lasers in the experimental lab. As the abbreviation for tryptophan is W, the protein used in the test was called BBAW.
Pande and Gruebele then set out with the goal of finding how long it would take the BBAW protein to unfold after a jump in temperature, as might occur if it was present in someone with a fever. This would be hard for Folding@home, as accurately measuring the time it takes a protein to fold had been a tough challenge for computers until that point.
Gruebele explains that back then "people were incredibly happy when their computer just ran one trajectory". He points out that the problem with this was that "if you run just one single simulation and watch it fold, one of several things might happen. It might not fold at all, as you can’t run it for long enough, or it might fold to an incorrect structure because your computer force field isn’t accurate enough, or it might fold after a specific amount of time, such as five microseconds".
As Gruebele says, this doesn’t actually tell you how fast the protein will fold in real life. "When you repeat this," he explains, "different proteins will take different amounts of time – some will be slower and some faster, and if you only have one computer observation, you don’t know whether that five microseconds is just one of the lucky ones that folds really fast."

MSI MPG Velox 100R Chassis Review
October 14 2021 | 15:04









Want to comment? Please log in.